I am the X-ray facilities manager for the Newcastle University MX Structural Biology group and collaborators. I joined Rick’s lewis laboratory in 2009.
hybrid two component system
Bacteria in the human gut utilise complex plant and host glycans as a major nutrient source. These bacteria play an important role in maintaining normal health and nutrition. In order to survive in this highly competitive environment, they must be able to sense and respond rapidly to the presence of carbohydrates in their dynamic environment. In Bacteroidetes, a dominant phylum in the gut, genes encoding proteins involved in complex glycan acquisition and degradation are arranged in clusters on the genome that are closely linked to genes encoding extracellular sensor-regulators. One of the most ubiquitous classes of sensor regulator in the gut Bacteroidetes are hybrid two component systems, which contain all the domains of a classical two component system, but in a single polypeptide.
We have determined the structure of the periplasmic sensor domain of one such HTCS both in apo and ligand bound form. This large protein (~790aa) crystallised with 6 molecules in the asymmetric unit giving a P321 unit cell with approximate dimensions of a=b=340A, c=100. Screening of many crystals was required to identify a crystal of high enough quality to collect sufficient data to locate the 42 selenomethione residues present. The structure was solved using SHELX to a resolution of 3.5 A and the phases extended to a native data set to 2.6A. Crystals of the ligand bound form of the protein were also obtained and the structure solved by molecular replacement using individual domains from the apo structure. Each monomer contains two seven-blade beta-propellers, which reveal the as yet undescribed Pfam Reg_prop family and also one example of the also undescribed Pfam Y_Y_Y domain, which exhibits an Ig-type fold.
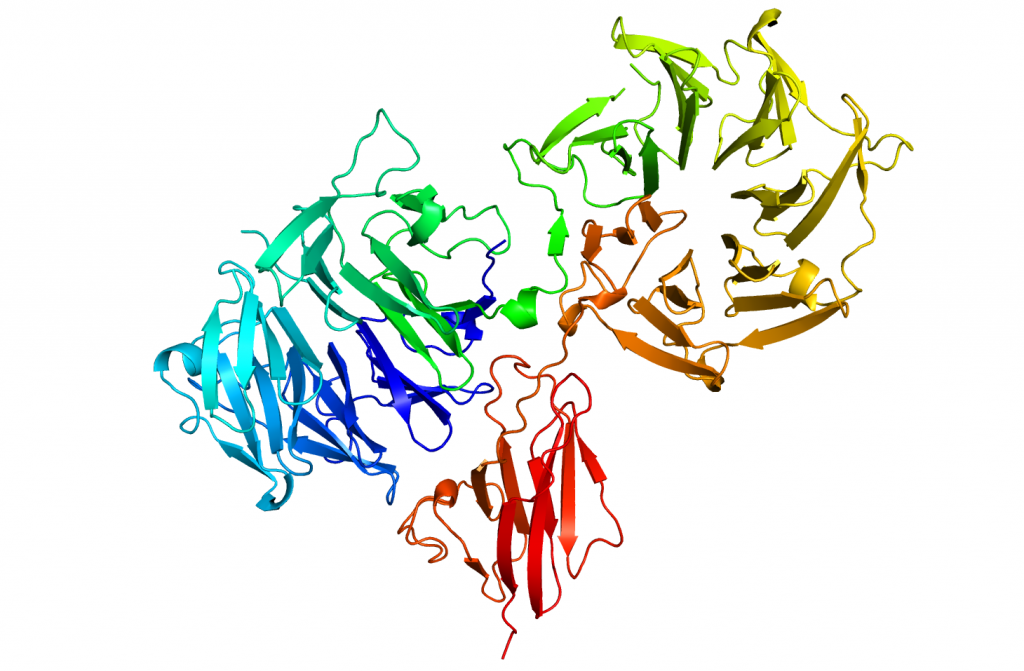
Lowe EC, Baslé A, Czjzek M, Firbank SJ, Bolam DN. (2012). A scissor blade-like closing mechanism implicated in transmembrane signaling in a Bacteroides hybrid two-component system. Proc Natl Acad Sci U S A. 109(19):7298-303. [Pubmed]
Collaborators: David Bolam (Newcastle University); Elisabeth Lowe (Newcastle University).
Methanobactin
Methanobactins(mbs) are small copper-binding molecules that are involved in the copper uptake system of the methane-oxidising bacteria. We have previously structurally characterized the mbs of Methylosinus trichosporium OB3b. The variability of the structures and properties of mb is not known. It is a key issue in understanding the role of methane-oxidizing bacteria in greenhouse gas suppression. We present here two new structures of mbs from Methylocystis stains M and hirsuta CSC1. Both molecules possess very similar hairpin-like structures which are very different to the more compact arrangement of the M. Trichosporium OB3b mbs.
The geometry of the copper sites is also conserved in mbs. The petide backbones are composed only of 4 standard amino acids and of two modified residues which include oxazolone and pyrazinedione rings. The main differences between the two mbs are the N- and C- termini. The most important of these is the presence of either a thioether or guanindine group at the N-terminus (figure 2A and 2B). The positively charged guanidine group of the Methylocystis strain M mb hydrogen bonds to the Cu-coordinating sulfur. This hydrogen bond decreases the electron density on the coordinating sulphur favouring the Cu(I) over the Cu(II)-form. The Methylocystis mbs are stabilized by the sulfonate group and its absence lowers the Cu(I) affinity approximately 30-fold (figure 3B, red dash line). We have identified and characterized two novel mbs. We postulate that mbs variation in their amino acid composition may be important for recognition and uptake.
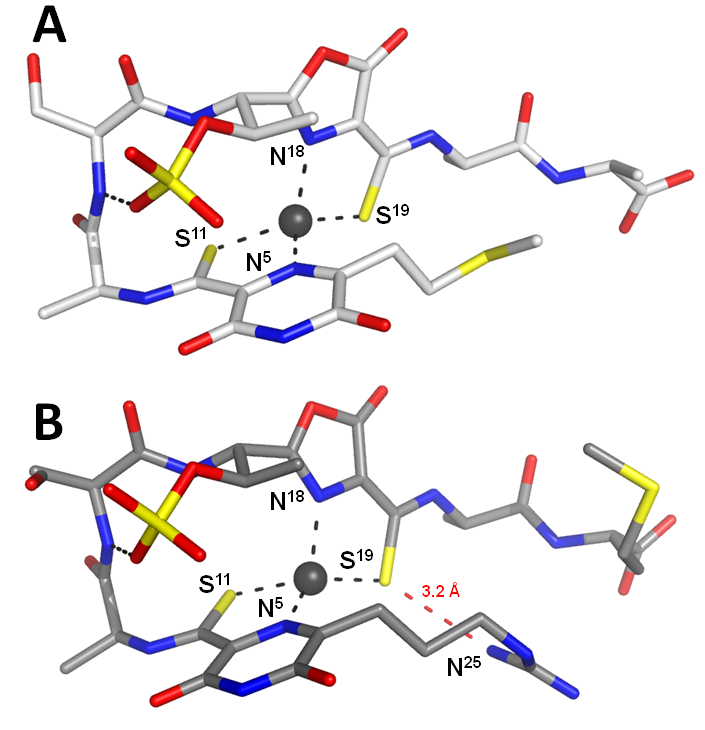
El Ghazouani A, Baslé A, Gray J, Graham DW, Firbank SJ, Dennison C. (2012). Variations in methanobactin structure influences copper utilization by methane-oxidizing bacteria. Proc Natl Acad Sci U S A. 109(22):8400-4. [Pubmed]
El Ghazouani A., Baslé A., Firbank S.J., Knapp C.W., Gray J., Graham D.W., Dennison C. (2011) Copper-binding properties and structures of methanobactins from Methylosinus trichosporium OB3b. Inorg Chem. 50(4):1378-91. [Pubmed]
Collaborators: Christopher Dennison (Newcastle University).